Understanding Herceptin Biosimilars
Herceptin biosimilars market are biologic medicinal products that closely mirror Herceptin (trastuzumab), a pioneering monoclonal antibody utilized in the treatment of HER2-positive breast cancer and other HER2-overexpressing malignancies. These biosimilars are designed to match the safety, purity, and potency of the original Herceptin, ensuring comparable efficacy in clinical outcomes while providing a cost-effective alternative.
Click here to get a Sample report copy@ https://www.amecoresearch.com/sample/277028
Market Benefits
Cost Reduction: Herceptin biosimilars offer a significant reduction in treatment costs, which is crucial in making cancer care more accessible to a broader patient population. The reduced price of biosimilars compared to the reference biologic can lower healthcare expenditure and improve patient adherence to treatment.
Increased Access: By providing a more affordable option, biosimilars enhance accessibility to effective cancer treatments, particularly in emerging markets where the cost of original biologics may be prohibitive.
Competitive Market Dynamics: The introduction of multiple biosimilars stimulates competition, which can drive innovation and further reduce costs, benefitting both healthcare systems and patients.
Extended Treatment Options: The availability of biosimilars contributes to a diversified portfolio of treatment options, allowing healthcare providers to tailor therapies according to individual patient needs.
To Check Toc: https://www.amecoresearch.com/herceptin-biosimilar-market/toc/277028
Market Strategies
Regulatory Pathways: Navigating the complex regulatory environment is crucial for market players. Companies must adhere to stringent regulatory requirements for biosimilars, including rigorous testing to demonstrate similarity to the reference product. Strategic engagement with regulatory bodies can facilitate smoother market entry.
Strategic Partnerships: Collaborations between biosimilar manufacturers and research institutions can accelerate development and approval processes. Partnerships with established pharmaceutical companies can also enhance market reach and distribution capabilities.
Market Expansion: Targeting emerging markets with growing healthcare infrastructure offers significant growth opportunities. Companies should focus on regions with high unmet medical needs and increasing cancer prevalence.
Educational Initiatives: Raising awareness among healthcare professionals about the safety and efficacy of biosimilars is essential for their adoption. Educational programs and clinical trials showcasing real-world evidence can build confidence in biosimilar therapies.
Market Aspects
Innovation in Production: Advances in biotechnology and manufacturing processes contribute to the development of high-quality biosimilars. Innovations in production technology can improve efficiency, reduce costs, and ensure consistent product quality.
Competitive Landscape: The Herceptin biosimilar market is characterized by intense competition among pharmaceutical companies. Key players include Pfizer, Amgen, and Mylan, each striving to capture market share through differentiated product offerings and strategic pricing.
Regulatory Challenges: Biosimilars must undergo extensive clinical testing and regulatory scrutiny to ensure they meet the stringent standards set for safety and efficacy. Navigating these regulatory requirements can be challenging but is crucial for successful market entry.
Patient and Provider Acceptance: Acceptance of biosimilars among patients and healthcare providers is influenced by factors such as clinical efficacy, safety profiles, and cost-effectiveness. Building trust through transparent communication and robust clinical evidence is essential for widespread adoption.
Conclusion
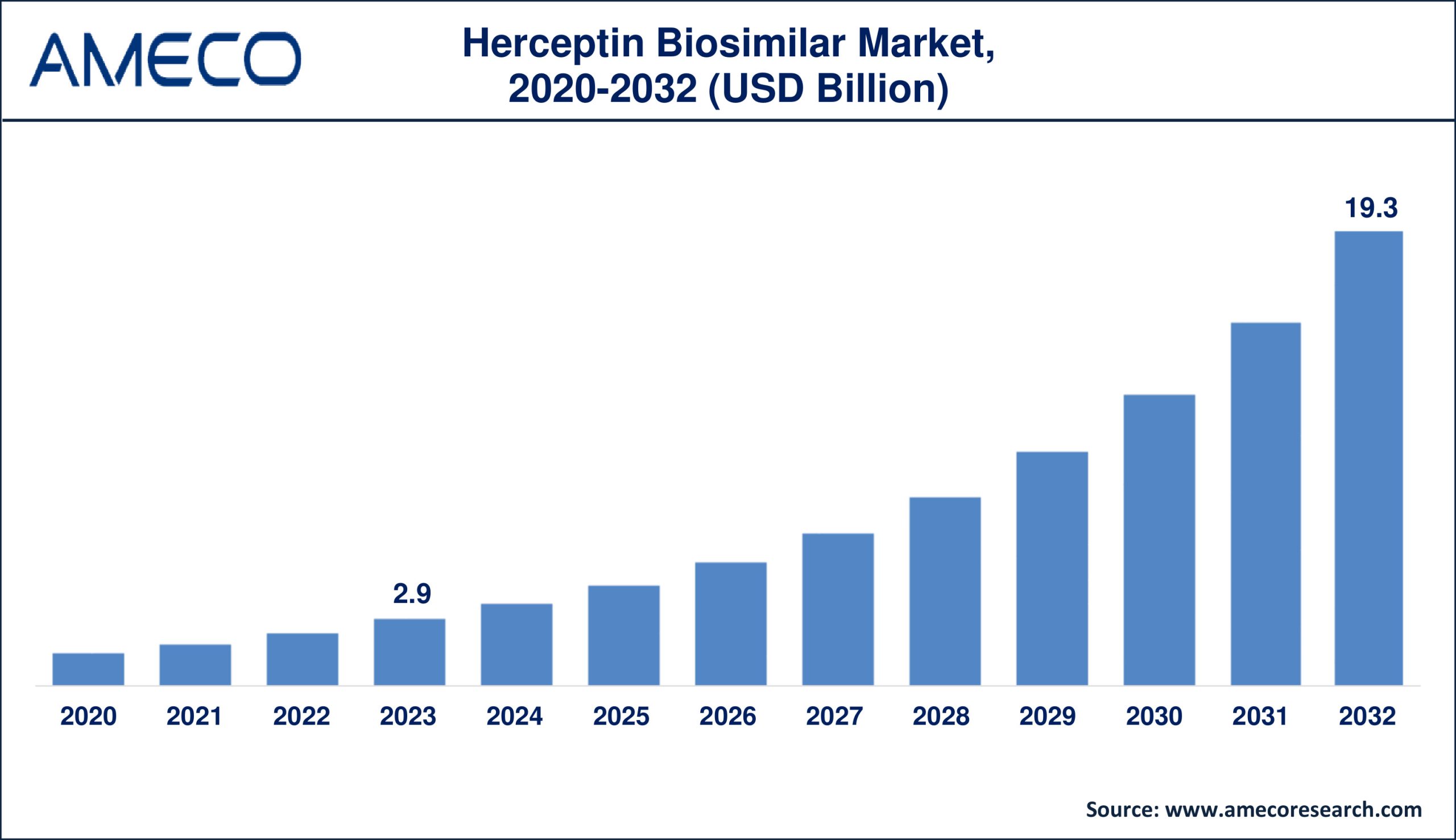
The Herceptin biosimilar market is poised for remarkable growth, driven by the increasing need for cost-effective cancer treatments and the ongoing advancements in biotechnology. As the market expands, stakeholders must navigate regulatory landscapes, foster strategic partnerships, and drive innovation to capitalize on the opportunities presented by this burgeoning sector.
MARKET SEGMENTATION:
Herceptin Biosimilar Market By Application
- Breast Cancer
- Colorectal Cancer
- Leukemia
- Lymphoma
- Others
Herceptin Biosimilar Market By End-User
- Hospital & Clinics
- Oncology Centers
- Others
CUSTOMIZED REQUIREMENTS? NEED ANY HELP? PLEASE EMAIL US @ sales@amecoresearch.com
To Purchase this Premium Report@ https://www.amecoresearch.com/buy/277028
About Ameco Research:
The complete information about our alliance publishers and the business verticals they cater to helps us in appropriately responding to our client requirements and identifying the potential opportunities in the market and suggest the research that can best suit client’s requirement. Our comprehensive list of research reports boasts a complete collection of database casing almost every market category and sub-category.
Browse For More Related Reports:
https://www.linkedin.com/pulse/herceptin-biosimilar-market-insights-forecast-till-2032-6jfmf/
The Global Healthcare PPE Market valued for USD 41.5 Billion in 2021 and is anticipated to reach USD 22.7 Billion by 2030 with a CAGR of 4.8% from 2022 to 2030.
The Global Drug Eluting Stent Market Size valued for USD 7.2 Billion in 2021 and is anticipated to reach USD 17.7 Billion by 2030 with a CAGR of 7.6% from 2022 to 2030.
The Global Orthopedic Devices Market Size valued for USD 38.6 Billion in 2021 and is anticipated to reach USD 59.7 Billion by 2030 with a CAGR of 5.1% from 2022 to 2030.
For Latest Update Follow Us on Twitter and, LinkedIn
Contact Us:
Mr. Richard Johnson
Ameco Research
India: +918983225533
E-mail: sales@amecoresearch.com
